If you or a loved one was injured after using an IVC filter you deserve compensation. Learn how the IVC filter lawyers at Babin Law, LLC may be able to represent you in your Columbus IVC filter claim.
When products are released on the market, many of them have FDA approval. But the truth is, the FDA does not test every product because they don’t have the time to do so. They leave the testing to the pharma companies who may test inadequately or provide incomplete findings to push their products through.
As a result, some products sold in drug stores and available by prescription may not produce results as advertised. Some may even be harmful to health. Inferior vena cava (IVC) filters have been coming under investigation for a defective design that has resulted in serious personal injuries.
What are IVC Filters?
IVC filters are small devices that resemble a cage. They are inserted into the inferior vena cava. This is the largest blood vessel in the body. It plays a key role in in moving blood from the lower half of the body to the heart and lungs.
The filters are implanted in the vein directly below the kidney. They are made to capture blood clots to keep them from reaching the lungs, a potentially fatal situation. They reduce the risk of blood clots, deep vein thrombosis and pulmonary embolisms.
Although IVC filters are made to reduce the risk of blood clots, it has been found that, in some cases, they have exactly the opposite effect. They may increase the risk of blood clots in some patients.
IVC filters are recommended for people at risk for developing blood clots in their legs. These include the following:
- People who have pulmonary embolisms
- People who have experienced trauma
- People who are immobile
- People who have recently had surgery
- Women who have recently had a baby
- People who have deep vein thrombosis
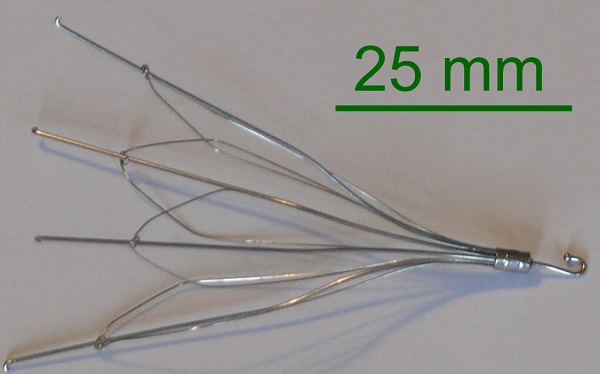
What is the IVC Issue?
IVC filters are dangerous because they have been known to break and move metal fragments throughout the blood causing organ damage. They have also been known to cause punctured veins and people have even died when using the product.
A product that is potentially dangerous should have warning labels that notify users of possible risks. However, IVC filter manufacturers did not put any warnings on the package relating to risks of breakage, organ damage, blood clot formation, or any sort of health condition.
Furthermore, it is alleged that the manufacturer knew of the risks associated with the product and hid the results from the public. Some allegations state the manufacturer forged an employee’s signature on the application in order to get FDA approval.
What IVC Products are Involved in Columbus Lawsuits?
There are many IVC products that have been targeted in lawsuits. These include the following:
- The Bard G2 Filter
- The Cook Celect Filter
- The Bard Recovery Filter
- The Bard G2 Express Filter
- The Cook Gunther Tulip Filter
- The Bard Eclipse
- The Bard Denali
- The Bard Meridian
How Bad Are IVC Filters in Columbus?
About 500,000 IVC filters are implanted in Americans every year. This number has gone up due to the creation of retrievable IVC filters that can be removed after the risk of embolism decreases. However, one study has revealed that the devices are only removed a third of the time.
Because the product is so dangerous, thousands of lawsuits have been filed. Plaintiffs have complained of the following issues:
- Filter fracture
- Difficulty removing the device
- Filter migration
- Perforation of the inferior vena cava blood vessel
- Embolization
- IVC occlusion
- Lower limb deep vein thrombosis
Bard IVC Filters
C.R. Bard has produced two filters that caused health issues: the Bard Peripheral Vascular Recovery and its G2 filter system. A 2010 study evaluated the company’s filters designed between 2004 and 2009.
Despite the company attempting to make repairs on their products, the study found 12% of devices had filter fractures, 16% had strut fractures, and 25% were embolized. There was also an FDA concern that short-term placement devices were not removed once the risk of embolism diminished.
The FDA sent a warning letter to the company in 2015 for not following quality regulations regarding its two cone removal systems used to remove IVC filters. The administration found these devices did not meet the requirements for clearance and premarket approval.
Cook IVD Filters
Cook Medical is another company dealing with issues due to faulty IVC filter designs. Their Gunther Tulip and Celect IVC Filters were found to have a risk of tilting, migration, and vein perforation. The longer the device was in the body, the greater the risk of perforation.
A 2014 study looked at additional perforation statistics for the Celect filters and found that 39% penetrated the vein within 30 days. 80% penetrated the vein within 90 days. 13% of devices were found to have punctured either the aorta, muscle, intestines, adrenal gland, liver, lymph node, pancreas, spine, kidney, or diaphragm.
This led to a lawsuit that claimed that Cook’s IVC filters were insufficiently tested and caused risks that outweighed their benefits.
How a Columbus IVC Filter Attorney Can Help With Your Claim
A medical product defect lawsuit is often complex. The plaintiffs will be going up against big businesses and will need medical evidence to support their claim. Having a reliable Columbus IVC filter lawyer on your side can help ensure that you have the evidence you need to get properly compensated.
At Babin Law, LLC, our Columbus personal injury lawyers have years of experience representing Columbus clients in product defect cases. We are known for our deep level of care and respect, our affordable rates, and our winning results. We will stop at nothing to see to it that justice is served.
Don’t let big pharma get away with releasing products that are not properly tested. Contact us to schedule a free consultation. If we’re able to take on your case, we will work to get you the compensation you deserve for your Columbus IVC filter personal injury lawsuit.


